Confederated Tribes of the Colville Reservation
Surface Waters Monitoring Program
Joe Peone, Program Director
Gerry Marco, Fishery Biologist

The Littoral Community of Buffalo
Lake: Changes in Macrophytes as an Indication of Trophic Trends with Implications
to the Lake Fishery
By
Judith Loescher
Edmond Broch
Department of Zoology
Washington State University
1 June 1996
(17 September
1996 Revision)
The
Littoral Community of Buffalo Lake: Changes in Macrophytes as an Indication of
Trophic Trends with Implications to the Lake Fishery
By
Judith Loescher
Edmond Broch
INTRODUCTION
Buffalo
Lake, a 218.6 ha closed basin, freshwater lake on the
Colville Indian Reservation, has
supported abundant growths of aquatic macrophytes in recent years. Harkness, et
al. (1974) reported dense growths of an unidentified species of milfoil (Myriophyllum
sp.) growing in a band around the lake. In late summer the plant was a nuisance
to boating and swimming activities near the shore.
As a result of reports of suspected
Eurasian watermilfoil (Myriophyllum spicatum L.) populations, a
1983 survey (Broch, Ahl and Loescher, 1983) of Buffalo Lake was conducted. It
confirmed the presence of Eurasian watermilfoil while identifying macrophyte
distribution in the lake. A follow-up survey was conducted in 1984 to determine
the direction and extent of Myriophyllum spicatum in Buffalo Lake
(Broch and Loescher, 1984).
In 1994, while collecting water samples
for the continuing monitoring of Buffalo Lake, Edmond Broch observed changes in
the aquatic macrophyte populations of the lake. Therefore, on July 17-18, 1995,
we conducted a survey of the macrophytes with particular attention to the
abundance and condition of Myriophyllum spicatum in the lake.
The Colville Confederated Tribes Monitoring
Program provides an extensive database which may shed light on the causes of
any change in the macrophyte community as well as on possible changes in the
trophic nature of Buffalo Lake. In addition the existence of such a database
provides an unusual opportunity to interpret changes in the population
structure of Eurasian milfoil within the macrophyte community since the 1984
study.
The database of the Limnology of the
Lakes of the Colville Reservation is available on the World Wide Web. The entire
database can be viewed at URL (). The
Database pertaining to Buffalo Lake can be viewed at .
METHODS
The aquatic plant community was sampled
with a spring steel rake head attached to a 15 m Dacron line. The rake was
dropped into the water from an Avon SR-4 hard-bottomed inflatable boat equipped
with a motor along transects established in earlier aquatic macrophyte studies
(Broch, Ahl and Loescher, 1983; Broch and Loescher, 1984), samples were taken
at meter depth intervals from depths ranging from 2 to 9 m. Sampling was done parallel
to shore along a single depth contour with the boat moving at approximately 2
knots, a speed slow enough to keep the rake dragging on the lake bottom.
Sampling depths were measured by Sonar using a Lowrance X-16 and Lowrance 350A
depth sounders. The rake was also used to retrieve plant materials from sites
not on the established transects. The map of Buffalo Lake (Fig. 1) shows the
locations of the sites sampled and the transect locations.
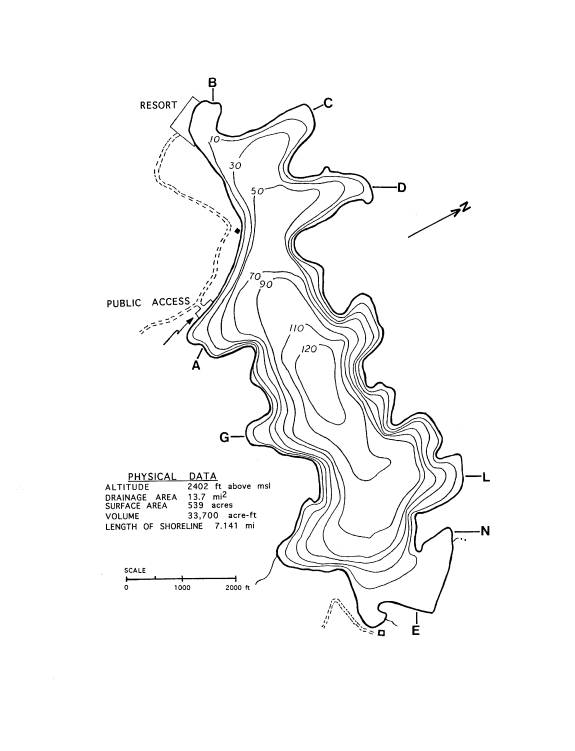
Figure
1. Map of Buffalo lake showing sampling locations (Depth contours are given in
feet.)
After collection, the macrophytes were
bagged, labelled, and placed in an ice chest for transport to the laboratory.
There they were identified within a few days using Hitchcock and Cronquist
(1973), Ceska and Ceska (1986), and Imahori and Wood (1965) as primary
resources.
After collection, the macrophytes were
b
Pressed plant material was deposited in
the Marion Ownbey Herbarium of Washington State University, Pullman,
Washington.
Measurements of temperature and oxygen
concentration were made using a Yellow Springs Instrument Co. Model 58
temperature/dissolved oxygen probe. Secchi disk transparency readings were made
at Stations A and B, the same stations used in the ongoing (1990 to present)
nutrient monitoring study conducted by Broch.
RESULTS
The aquatic macrophytes found in
Buffalo Lake, together with the locations and depths at which each was found,
are presented in Table 1 below:
TABLE 1. BUFFALO LAKE TRANSECT SITES BY BAYS.
Data indicates
locations (bays) and depths (in meters) at which macrophytes and macroalgae
were found. Aquatic plants were not found in transects from bays B, G, and M.
|
|
|
|
|
|
|
|
|
|
A |
C |
D |
E |
L |
N |
|
macroalgae |
|
|
|
|
|
|
|
Chara sp. |
<0.3 |
1-1.2 |
|
|
1.5-2.0 |
1.0 |
|
Nitella sp. |
<0.3 |
1-2.0 |
1.2 |
1-2.0 |
1.5-2.0 |
|
|
unidentified charophytes |
|
1.6 |
|
1-2.0 |
|
|
|
Spirogyra sp. |
|
2.0 |
|
|
|
|
|
emergents |
|
|
|
|
|
|
|
Eleocharis acicularis L. (R. & S.) |
|
1.2 |
|
|
|
|
|
Eleocharis palustris L. (R. & S.) |
|
<1-2.0 |
|
|
|
|
|
Glyceria sp. |
|
<1-2.0 |
|
|
|
|
|
Polygonum sp. |
<1 |
|
|
|
|
|
|
Sagittaria cf. teres
Wats. |
|
|
|
1.0 |
|
|
|
Scirpus acutus Muhl. |
|
<1-2.0 |
|
|
|
|
|
submersed macrophytes |
|
|
|
|
|
|
|
Ceratophyllum demersum L. |
|
|
|
|
2.0 |
1.0 |
|
Elodea canadensis Rich. |
|
<1-1.6 |
|
0.7-2.0 |
2.0 |
1.0 |
|
Elodea cf. nuttallii (Planch.) St. John |
|
|
|
1.0 |
|
|
|
Myriophyllum sibiricum Kom. |
|
<1-1.2 |
|
0.7-2+ |
2.0 |
1.0 |
|
Najas flexilis (Willd.) R. & S. |
|
1.6 |
|
|
|
|
|
Potamogeton sp. |
|
|
<1-1.0 |
|
|
|
|
Potamogeton foliosus Raf. |
|
<1-1.6 |
|
0.7-2.0 |
|
|
|
Potamogeton gramineus L. |
|
|
|
1.0 |
|
|
|
Potamogeton pectinatus L. |
|
|
|
0.7-1.0 |
|
|
|
Potamogeton praelongus Wulf. |
|
|
|
2+ |
2.0 |
|
|
Potamogeton pusillus L. |
|
|
|
0.7-1.0 |
|
|
|
Potamogeton richardsonii (Benn.) Rydb. |
|
1-1.2 |
|
1.0 |
|
|
|
Potamogeton robbinsii Oakes |
|
|
|
|
2.0 |
1.0 |
|
Potamogeton zosteriformis Fern. |
|
1.2 |
|
1-2.0 |
2.0 |
1.0 |
|
Ranunculus aquatilus L. |
|
|
|
|
<1-2.0 |
|
|
Tillaea aquatica L. |
|
1.2 |
|
|
|
|
|
Zannichellia palustris L. |
|
|
|
0.7 |
|
|
. The results of sampling, presented by
bay and transect, follow.
Bay A - Public Launch Bay
At the shore, stems of last year’s growth
of the terrestrial plants flannel mullein (Verbascum lanatum) and
knapweed (Centaurea sp.) were inundated by approximately 0.6 m of water,
indicating that the lake water level was higher at the time of sampling than
when those plants were growing.
Transect A3
No living aquatic plants were found in
either a long tow extending from 2 m to 9 m water depth or from individual
throws at the 2 m and 3 m depths.
Transect A4
No living plant material was found in
either a long tow extending from 2 m to 9 m depth or from discrete sampling at
the 2 m through 4 m depths.
With the exception of small plants of
the charophytes Nitella sp. and Chara sp. in <0.3 m water, and
a single emergent plant of Polygonum sp., no living plants were found in
Bay A. Even the concrete launch ramp was devoid of visible growths of algae
throughout its submerged length. A small amount of brown, decaying Potamogeton
and other plant detritus was found in the samples. Amphipods were present in
the collected charophytes. Several small (<2.5 cm) crayfish were observed
among the charophytes, dead terrestrial plant material and rocks in shallow
(<0.3 m) water near the launch ramp.
Bay B - Reynolds Resort Bay
No aquatic plants were found except for
the emergent macrophytes which were particularly abundant along the northwest
shore of the bay. Samples from 1.0, 1.5, 2.0, 3.0 and 5.0 m on Transect B1
yielded only brown, decaying plant detritus.
Bay C
The emergent aquatic plants Scirpus
acutus (hardstem bulrush), Eleocharis palustris (slender
spikerush) and Glyceria sp., a grass, were present at the north end of
Bay C and in samples from <1 m? to 2
m. At <1.0 m, dense growths of Elodea canadensis approached
the water surface. The plants were about to bloom. One strand of Potamogeton
foliosus was found. A second sample contained Myriophyllum sibiricum.
The bottom sediments were mucky. Plant detritus was found in samples from 3 m
and 4 m, but no living plants were found.
A band of emergent macrophytes about 7
m - 9 m long extended about 6-8 m from shore.
In the first of 2 samples from 1 m taken just off the band of emergents
only a handful of living macrophytes was retrieved. Elodea canadensis
and Potamogeton richardsonii, together with a few sprigs of Potamogeton
foliosus and a young grass rhizome were identified. The other sample
from 1 m contained only a small amount of the charophytes Nitella sp.
and Chara sp.
A sample from 1.2 m contained Potamogeton
foliosus, Elodea canadensis and fragments of Potamogeton
richardsonii, Potamogeton zosteriformis, Myriophyllum
sibiricum, Tillaea aquatica, and the charophyte Nitella
in fine organic sediments.
A sample from 1.6 m contained Potamogeton
foliosus and Elodea canadensis in roughly equal amounts, a
strand of Najas flexilis and a small amount of the charophyte Nitella
sp.
A sample from 2 m contained only a
small amount of Nitella sp., growing in organic sediments.
A collection from 1.5 m in the western
part of the bay yielded a handful of Nitella sp.
Bay D
Emergent aquatic plants were present at
the north end of the bay. A band of Potamogeton sp., about 10-14 m long
and about 3.3 m wide (interrupted in one area), extended from the shore to a maximum depth of about 1 m.
Samples were taken from 1.2 m through 8
m depths in Bay D. The 1.2 m collection contained only the charophyte Nitella
sp. Amphipods were present. Collections from 2 m and 3 m contained only fine
detritus. The substrate was silty with fine organic matter. Samples from deeper waters contained only
plant detritus.
Bay E
Emergent aquatic macrophytes were
present along the inflowing stream and along the shore of the lake. Submerged
aquatic macrophytes densely covered the lake bottom out to the 2+ m depth
level. At the outer limits of plant colonization, the lake bottom was covered with
fairly solid turf of Elodea canadensis with individual plants of Myriophyllum
sibericum interspersed at approximately 1 to 2 m intervals. In slightly
shallower water were dense beds of Myriophyllum sibiricum. The
tips of the plants had reached the water surface and were in flower. Inshore
there were large, dense beds of Potamogeton pectinatus, also in
flower. Small colonies of Potamogeton praelongus were also
visible.
A grab sample from 2±m contained one
strand each of Potamogeton praelongus and Myriophyllum sibericum.
A collection (E2mE) from 2m from the
eastern area of the bay yielded Elodea
canadensis and Nitella sp. along with a sprig of Myriophyllum
spicatum.
Collection E1mN from 1 m (about 7.2 m
off the emergents) contained a moderate amount of macrophytes including Potamogeton
pusillus (64%, Elodea cf. nuttallii (16%) and Myriophyllum sibiricum (23%)
growing on organic silt.
Collection E2mW from 2 m contained a
moderate amount of plant material including Chara? sp.(30%), Nitella
sp.(30%), Potamogeton foliosus (20%), Myriophyllum sibericum
(20%), and a sprig of Potamogeton zosteriformis (5%).
A large collection (E0.7m#2) from 0.7 m
contained 50% Myriophyllum sibericum, 30% Elodea canadensis,
10% Potamogeton pusillus, 8% Potamogeton pectinatus
and a few plants of Potamogeton foliosus and Zannichellia palustris
on organic sediments.
A moderately large collection from 1 m
(E1mE) contained 60% Potamogeton richardsonii and 15% Potamogeton
foliosus, 15% Potamogeton pectinatus (about to flower), 5%
Elodea canadensis, and a sprig or two each of Potamogeton gramineus,
Potamogeton zosteriformis and Myriophyllum sibericum.
Also present were Nitella sp., a plant of Sagittaria cf. teres, and an unidentified charophyte. The
substrate was silty sand with fine organic matter.
Bay G
Emergent aquatic plants were at the
shore, but no submerged plants were found along Transect G2 except for a
charophyte fragment from 1 m.
Bay L
A Transect L sample from <1m
contained a moderate amount of Ranunculus aquatilus in flower. A
collection from 1.5 m contained a small amount of plant material including Ranunculus
aquatilus (in early fruit) and the charophytes Chara sp. and Nitella
sp.
A collection from ±2 m contained a
small amount of plant material. Elodea canadensis comprised 90%
and Myriophyllum spicatum and Potamogeton zosteriformis
each made up 5%.
A collection from 2 m (L2-2) contained Potamogeton
robbinsii, Potamogeton praelongus, Myriophyllum sibiricum,
Elodea canadensis, and a small amount of the charophytes Chara
and Nitella. Sediments were fine
and organic. An additional collection from 2 m contained a moderate amount of Elodea
canadensis, together with a couple strands each of Ceratophyllum demersum,
Potamogeton robbinsii, Ranunculus aquatilus and Myriophyllum
sibericum.
Bay N
A collection from 1 m contained a
moderate amount of plant material including Myriophyllum sibericum,
Elodea canadensis, Ceratophyllum demersum, Potamogeton
robbinsii, Potamogeton zosteriformis and a sprig of Chara,
all growing on organic sediments.
DISCUSSION
Nomenclature
of Myriophyllum exalbescens and M. sibiricum
The scientific name of the commonly
found native northern watermilfoil, Myriophyllum exalbescens
Fern., is now known as Myriophyllum
sibericum Kom. (Ceska and Ceska, 1986). Most literature refers to the
plant as M. exalbescens, but the reader is advised that the two
names refer to the same taxon.
Present
Survey
On July 17-18, 1995, the aquatic
macrophytes of Buffalo Lake were restricted to depths of approximately 2 m and
shallower. Buffalo Lake has experienced
a catastrophic decline in macrophytes, in abundance as well as in the extent of
colonization of the littoral zone. Both were severely reduced as compared with
the most recent macrophyte survey (Broch and Loescher, 1984).
Of the eight bays chosen for the 1995
study because each had had abundant aquatic vegetation, only three (C, E, and
L) were significantly colonized. Bay E,
in the easternmost end of the lake, contains the largest shallow area in the
lake. In 1995 as well as in 1983 (Broch, Ahl and Loescher, 1983) and 1984, it
also contained the greatest mass of aquatic plants.
In 1995, Bay E contained the greatest diversity (11
species) of submersed macrophytes. Bays C and L each had 7 taxa. Only charophytes and a single amphibious
knotweed (Polygonum sp.) plant were found in Bay A (near the public
launch). In Bay D, only Nitella sp. and
Potamogeton sp. were found. Only 5 submersed macrophytes and Nitella
sp. were found in Bay N, all at the 1 m depth. In bays B and G where
charophytes, Elodea canadensis and Myriophyllum spicatum
had been found in 1984, not a single macrophyte was found.
As surprising as the profound decline
in macrophytic biomass is the apparent total absence of Eurasian watermilfoil (Myriophyllum
spicatum). In place of M. spicatum, the native milfoil Myriophyllum
sibericum Kom. (= M. exalbescens Fern.) was found.
Otherwise, the taxa found in each of the surveys
have been fairly consistent.
Table 2 on the next page (p14) lists
the vascular macrophytes reported in the surverys of 1983, 1984, and 1985. Myriophyllum
spicatum, found in 1984 along all transects except that of Bay S, was
not identified in any of the 1995 collections, nor was a single M. spicatum
plant observed as we cruised the littoral zone of the lake. Except for the
replacement of Myriophyllum spicatum by M. sibericum
in 1995, the major taxa found in each of the surveys have been fairly
consistent. Presence or absence of the rarer taxa in one or more years might be
real or the result of chance sampling.
Plants identified in the 1995
collections but not found in 1984 are Najas flexilis, Potamogeton
foliosus, Potamogeton pectinatus, Potamogeton richardsonii,
Potamogeton zosteriformis, and Tillaea aquatica.
All but Potamogeton zosteriformis, Najas flexilis
and Tillaea aquatica were found in 1983. The potamogetons and Najas
are all commonly found plants while the tiny T. aquatica is
classified as a sensitive plant by the Washington Natural Heritage Program
(1990). A plant is labeled sensitive when it is vulnerable or declining. Such a
plant could become endangered or threatened in the state unless threats
TABLE 2. COMPARISON OF VASCULAR AQUATIC MACROPHYTES FOUND
IN 1983*, 1984**, AND 1995 SURVEYS OF BUFFALO LAKE
|
|
|||
|
|
|
|
|
|
|
1983 |
1984 |
1995 |
|
Ceratophyllum
demersum |
x |
x |
x |
|
Eleocharis
acicularis |
x |
x |
x |
|
Eleocharis
palustris |
x |
x |
x |
|
Elodea
canadensis |
x |
x |
x |
|
Elodea
nuttallii |
x |
x |
x |
|
Glyceria
sp. |
|
|
x |
|
Isoetes
sp. |
x |
|
|
|
Juncus
balticus |
|
x |
|
|
Limosella
aquatica |
x |
x |
|
|
Myriophyllum
sibericum (=M. exalbescens) |
|
x |
x |
|
Myriophyllum
spicatum |
x |
x |
|
|
Najas
flexilis |
x |
|
x |
|
Polygonum
amphibium |
x |
x |
|
|
Polygonum
coccineum |
x |
|
|
|
Polygonum
sp. |
|
|
x |
|
Potamogeton
alpinus |
x |
|
|
|
Potamogeton
foliosus |
x |
|
x |
|
Potamogeton
gramineus |
x |
x |
x |
|
Potamogeton
pectinatus |
x |
|
x |
|
Potamogeton
praelongus |
x |
x |
x |
|
Potamogeton
pusillus |
x |
x |
x |
|
Potamogeton
richardsonii |
x |
|
x |
|
Potamogeton
robbinsii |
x |
x |
x |
|
Potamogeton
zosteriformis |
|
|
x |
|
Potamogeton
sp. |
|
|
x |
|
Ranunculus
aquatilus |
x |
x |
x |
|
Tillaea
aquatica |
|
|
x |
|
Zannichellia
palustris |
|
x |
x |
|
|
|
|
|
|
* from Broch, Ahl & Loescher (1983) |
|
|
|
|
** from Broch & Loescher (1984) |
|
|
|
to its existence are removed or management programs are
established to ensure its existence.
Two shallow water macrophytes, Juncus
balticus and Limosella aquatica, found in 1984 were not
found in the 1995 survey. Each had occupied depths of <1 m in one or two
sites. These might have been present in isolated very shallow sites that were
not sampled.
Depth of Colonization
In 1995, aquatic macrophytes were
restricted to depths of approximately two meters and shallower. In contrast, in
1984 aquatic plants colonized Buffalo Lake to a maximum depth of 9 m. In 1984, Elodea
canadensis was the dominant macrophyte in approximately 40% of the
littoral zone of Buffalo Lake. Myriophyllum spicatum was dominant
in about 30% of the littoral zone. The remaining 30% was occupied by a variety
of shallow zone macrophytes and patches of the deepwater potamogetons P.
praelongus and P. robbinsii.
In 1984 Myriophyllum spicatum
was found along all transects except the Bay S transect which contained only Elodea
canadensis and charophytes. Myriophyllum spicatum
colonized the 2-5 m depth zone and was the most abundant plant at depths from 3
m to slightly less than 5 m. Elodea canadensis was found at some
depth in all of the 24 transects which were distributed among 17 embayments. E.
canadensis dominated the 5-9 m depth zone.
Possible
Causes for the Decline of Aquatic
Macrophytes in Buffalo Lake
A reasonable although tentative
explanation for the decline of aquatic macrophytes in Buffalo Lake can be
formulated from (1) data from previous macrophyte studies on Buffalo Lake, (2)
observations during lake monitoring, (3) the extensive limnological database on
the lake and (4) reports in the literature on causes of macrophyte declines in
other lakes.
There appears to be a shift in the
primary productivity dominance between the littoral macrophytes and limnetic
phytoplankton of the lake. Low Secchi disk transparency readings of 3 m, 4.5 m,
and 2.5 m recorded on April 20, 1993, September 9, 1993, and April 18, 1995,
respectively with concurrent reduction in macrophyte production below 2.5
meters indicate light as a possible
limiting factor. The shading produced by increased population density of algae
would inhibit the growth of macrophytes. Since the macrophytes found in 1995
were almost completely restricted to the 0-2.5 m depth zone of the littoral
where the shading effect of algae would be minimal, shading by high
phytoplankton populations is strongly suggested.
The phytoplankton dominance effect on
macrophyte abundance and distribution is not unusual in lakes on the eastern
side of the Colville Reservation. Both Little Goose Lake and Owhi Lake have
macrophyte productivity limited to
depths of less than 3 meters as a result of phytoplankton shading. In these
cases heavy blue green algal blooms are responsible for the shading affect.
The key question is what conditions
have brought about this rather abrupt and significant change in the primary
productivity dominance of Buffalo Lake?
Increases or changes in nutrient levels
and/or ratios may favor phytoplankton over macrophyte productivity. Any slight
competitive advantage resulting in increased production of phytoplankton would
be further enhanced by an increase in shading. The shading would be most
critical at depths greater than two to three meters.
Whether the changes in the increased
shading by phytoplankton is primarily quantitative due to a simple increase in
overall primary productivity or whether there has been a qualitative change in
species composition of the phytoplankton resulting in greater shading affect is
not known. An earlier study of Buffalo
Lake phytoplankton could be used to assess possible changes in the structure of
the phytoplankton community. The impact on zooplankton would be of great interest to fishery
management of Buffalo Lake.
Buffalo Lake water quality monitoring
data from the past 5 years show no readily apparent changes in nutrient
availability though a more rigorous analysis is underway. The possibility of
nutrient changes should not be discounted. There are other indications of
nutrient changes in Buffalo lake. There is no knowledge of any great physical changes occurring in
the lake recently.
There is evidence of increased
productivity (eutrophication) within Buffalo Lake by the decline in oxygen
levels within the hypolimnion of the lake as shown by monitoring data. Buffalo
lake nutrient and oxygen-temperature profile data can be accessed through the
following World Wide Web address: .
A comparison of the oxygen-temperature
profiles toward the end of summer stratification from 1988 to 1995 shows a gradual but consistent increase in the
thickness of the anoxic zone within the hypolimnion. The oxygen-temperature
profiles can be accessed by the following hyperlink addresses:
1995
1994
1993
1992
1991
1990
1989
1988
The increase in the anoxic zone from
< two meters (1990) to >7 (1993) meters takes place during the time of
decrease in macrophyte production. The years 1993 to 1995 coincide with the
loss of macrophyte production at depths less than three meters. The greatest
spread in range can be seen by looking at the oxygen profiles 1989 (lowest) and
1993 (highest). To what extent the increased organic productivity resulting in
reduction of oxygen within the hypolimnion is due to decay of macrophytes and/or
to increased phytoplankton productivity is not known. However the latter
hypothesis is a better fit of the data.
Continued monitoring of nutrients and especially oxygen levels may give
some idea of the factors responsible.
Other factors, such as poor growing
conditions for macrophytes (e.g., cool water temperatures or adverse water
level fluctuations) might have permitted phytoplankton and/or periphyton
populations to become unusually dense. Continued monitoring of Buffalo Lake may
provide alternate hypothesis.
A primary question is this a stable
trend or a perturbation with a return to prior conditions? Continued monitoring
is essential to determine whether this is a real change in the limnology of
Buffalo Lake. Buffalo Lake may alternate between phytoplankton and macrophyte
dominance. Only continue monitoring will provide answers to this important
question.
Herbivory and non-consumptive clipping
by crayfish have been reported to markedly reduce macrophyte biomass in lakes
(Letson and Makarewicz, 1994; Lodge, 1991). The crayfish population in Buffalo
Lake has increased in recent years as evidenced by the 40 lb of the crustaceans
caught in the 1994 gill net survey (Gerry Marco, personal communication).
Although crayfish activities might have contributed to the macrophyte decline,
it is unlikely that they were the primary cause. Observations by SCUBA have
shown that crayfish activity occurs in the 1-3 m zone (Lodge, 1991). Throughout
Buffalo Lake numerous species of macrophytes have disappeared from depths
greater than 2.5 m. It is unlikely that populations of crayfish are either
dense enough or uniform enough to cause the demise of all plants below the 2+ m
depth in a lake as large as Buffalo. Also, crayfish are known to prefer certain
plants over others (Lodge, 1991).
Insect infestations (Creed and Sheldon,
1994; Kangasniemi, 1983; Painter and McCabe, 1988) and disease (Shearer, 1994)
have been implicated in declines of Myriophyllum spicatum,
usually after biomass of the weed had been at a high level for about 10 years.
Although disease organisms and herbivory by insects might have been factors in
the decline of Eurasian watermilfoil, they are unlikely to have affected other
taxa because they tend to be host-specific.
It is likely that the loss of aquatic
macrophytes from the 2.5 m and deeper area of littoral zone in Buffalo Lake is
due to a combination of factors, the primary factor being a shift in the
primary productivity dominance between the littoral macrophytes and limnetic
phytoplankton of the lake. The shift has resulted in increased shading by
phytoplankton and/or periphyton.
Implications of Aquatic Macrophyte Decline in Buffalo Lake
With the profound reduction in
macrophytes in Buffalo Lake, the nutrients that had been contained in plant tissues
will be released to the water as decay occurs. The increased levels of
available nutrients are likely to stimulate either a bloom of phytoplankton and
periphyton or increased growths of aquatic macrophytes. If the phytoplankton
populations remain very dense throughout the growing season, they will inhibit
the macrophytic recolonization of the Buffalo Lake littoral zone by shading.
The impact of loss of macrophytes for food and cover for aquatic insects and
fish fry is considerable.
Higher phytoplankton numbers may cause
decreases in the dissolved oxygen concentration within the hypolimnion during
summer stratification and during winter stratification as well. This hypothesis
appears to be supported by the monitoring data discussed earlier. It is important to closely monitor changes
in the anoxic zone during summer stratification. The oxygen-temperature
profiles from past monitoring have been extremely informative as to possible
changes in Buffalo lake.
The shift to phytoplankton dominance in
Buffalo Lake has important implications to management of the lake as an
important fishery. Qualitative and/or quantitative changes in
phytoplankton composition could have
enormous impact on the fishery by affecting zooplankton diversity and
abundance. Zooplankton studies are being initiated to ascertain affect on
plankton in Buffalo Lake.
APPENDIX
Summary of Aquatic Macrophyte Observations - 1983-1989
July, 1983
The aquatic macrophytes growing in
eight bays (labelled A-G) of Buffalo Lake were sampled. Myriophyllum spicatum
was the most abundant plant in the 2-4 m depth zone. The transition to
dominance by Elodea canadensis occurred in the 3.5-4 m depth
zone. Elodea canadensis or Elodea sp. was either the most
abundant plant or the only species present at the 5-9 m depths.
July 10-13, 1984
Myriophyllum spicatum
and Elodea canadensis formed two nearly continuous overlapping
bands around Buffalo Lake. Myriophyllum spicatum formed the band
at the 2-5 m depth. Elodea canadensis grew in deeper water, from
5-8 m and occasionally to 9 m. Myriophyllum spicatum was the
dominant species in the 3-4 m zone and Elodea canadensis was dominant in
the 6-9 m depths. The change in species dominance occurred in the 4-5 m zone. Myriophyllum
spicatum was found along all transects except transect S, while Elodea canadensis was found along every
transect. The other macrophytes found in Buffalo Lake had a patchy
distribution.
In 1984, 6 emergent macrophytes were
found, 13 submerged macrophytes, and several charophytes.
September 14, 1986
The water level in Buffalo Lake was
down approximately 1.3-1.6 m from 1984. In all grab collections from depths
greater than 3.5 m, Elodea canadensis was the dominant taxon. Myriophyllum
spicatum was dominant in all collections from 2-3 m. Myriophyllum
spicatum was present around the perimeter of the lake, forming a ring
interrupted for short distances in some steep, rocky areas. In shallow zones
flowering Myriophyllum spicatum formed an almost impenetrable
mass from the lake bottom to the surface.
May 5-6, 1987
Stands of Myriophyllum spicatum
were present. Vigorous growth was observed only basally and at the tips of
overwintered plants.
May 10, 1988
In Bay B, Myriophyllum spicatum was observed with tips of plants showing new
growth but older leaves in poor condition and covered with periphyton.
September 28, 1988
M. spicatum was blooming in
2-3.5 m water depths at Station L, at H; M. spicatum was present
at D, C, and P.
Emergent macrophytes Eleocharis
sp. and rushes were present at C, D, J,
E, F, as usual. Potamogeton praelongus was present in Bay B.
September 11, 1989
M. spicatum was still
abundant, though perhaps less abundant than before. Fewer plants reached the
water surface than in September of 1988. Much of the Elodea canadensis
was covered with algae. The elodea formed a solid understory. Also seen were
patches of Potamogeton praelongus, P. robbinsii and
a fine-leaved potamogeton, possibly P. pusillus.
In Bay B, M. spicatum
looked similar to before, flowering abundantly. Elodea canadensis
was still the understory plant, but it was not as visible as before. Potamogeton
praetensis beds were dense but the plants appear to be well-grazed,
possibly by snails. Leaves were yellowish, missing epidermis, some leaf
margins, and there were holes in the leaves.
Literature Cited
Broch, E., J.
Ahl, and J. Loescher. 1983. The distribution and abundance of aquatic
macrophytes in Buffalo Lake, Okanogan County, Washington. Report to Colville
Confederated Tribes. Aquatic Macrophyte Study. October 1983. Department of
Zoology, Washington State University. 13 p.
Broch, E., and
J. Loescher. 1984. The direction and extent of the Eurasian watermilfoil
invasion of Buffalo Lake, Okanogan County, Washington. Report to Colville
Confederated Tribes. Buffalo Lake Aquatic Macrophyte Study. December 1984.
Department of Zoology, Washington State University. 26 p.
Broch, E., and
J. Loescher. 1995. The Limnology of the Lakes of the
Colville
Reservation. World Wide Web address:
URL (). The
Database pertaining to Buffalo Lake can be viewed at .
Ceska,
Oldriska, and Adolf Ceska. 1986. Myriophyllum (Haloragaceae) in British
Columbia: Problems with identification.
Pp. 39-50. In: Lars W. J. Anderson, ed. Proceedings of the First
International Symposium on watermilfoil
(Myriophyllum spicatum)
and related Haloragaceae species. Aquatic Plant Management Society. 223 p.
Creed, Jr.,
Robert, P., and S. P. Sheldon. 1994. The effect or two herbivorous insect
larvae on Eurasian watermilfoil. J. Aquat. Plant Manage. 32: 21-26.
Harkness, R.
E., D. A. Myers, and G. C. Bortleson. 1974. Water Resources of the Colville
Indian Reservation, Washington. Open File Report. United States Department of
the Interior Geological Survey. Tacoma, Washington.
Hitchcock, C.
L., and A. Cronquist. 1973. Vascular Flora of the Pacific Northwest. University
of Washington Press. Seattle. 730 p.
Kangasniemi,
B. J. 1983. Observations on herbivorous insects that feed on Myriophyllum
spicatum in British Columbia. pp. 214-218. In: Lake Restoration, Protection and
Management. Proceedings of Management Society. U.S. Environment Protection
Agency. Washington, D. C.
Letson, M. A.,
and J. C. Makarewicz. 1994. An experimental test of the crayfish (Orconectes
immunis) as a control mechanism for submersed aquatic macrophytes. Lake
and Reserv. Manage. 10(2): 127-132.
Lodge, D. M.
1991. Herbivory on freshwater macrophytes. Aquatic Botany 41: 195-224.
Painter, D.
S., and K. J. McCabe. 1988. Investigation into the disappearance of Eurasian
watermilfoil from the Kawartha Lakes. J. Aquat. Plant Manage. 26:3-12.
Washington
Natural Heritage Program. 1990. Endangered, Threatened and Sensitive Vascular
Plants of Washington. Olympia, Washington: Department of Natural
Resources. 52 p.
Wood, R. D.,
and K. Imahori. 1965. Monograph of the Characeae. Verlag von J. Cramer.
Weinheim.